Content
Introduction
Trade agreements shape the global agricultural trade landscape, encompassing both tariff and non-tariff measures. Among the latter, sanitary and phytosanitary (SPS) measures such as maximum residue limits (MRLs) are gaining attention. As trade tensions escalate, there is growing concern that countries could use SPS measures as a tool of retaliation in international agricultural markets, as they are often a focal point in trade negotiations (Disdier and Marette, 2010; Disdier and van Tongeren 2010; Grant et al., 2015).
MRLs set upper thresholds for residues of veterinary drugs, pesticides, and contaminants in food products, playing an increasingly central role in global trade dynamics (Gourdon et al., 2020). The process of establishing these national MRLs is required to follow a science-based risk assessment or aligned with international standards set by Codex Alimentarius (Codex). Codex sets veterinary drug MRLs based on evaluations conducted by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), with a primary emphasis on public health (FAO and WHO, 2009). However, the availability of scientific data is often limited, creating regulatory gaps. Given the lack of global consensus on what constitute risk assessment, MRLs for specific drugs can vary significantly across countries and products (Karemera et al., 2021).
Until the 1990s, MRLs posed minimal challenges to food trade (Yeung et al., 2017; Hejazi et al. 2021). The 2000s saw a regulatory shift when countries began adopting stringent, often unique MRL regimes. For example, Taiwan implemented a “positive list” in 1999, followed by Japan in 2006, and the EU’s harmonized system in 2008. Similar moves by China, South Korea, Hong Kong, and others have created a fragmented global regulatory landscape (Yeung et al., 2017). These shifts matter. Over 36% of SPS notifications to the World Trade Organization (WTO) involve MRLs, often stalling or complicating market access (Hejazi et al., 2021).
Veterinary Drug MRLs
Past research has primarily focused on pesticide MRLs (Li and Beghin 2011; Santeramo and Lamonaca 2019). In a new paper, we offer the first comprehensive cross-country study of veterinary drug MRLs (see Okunola et al., 2025). We developed a stringency index that captures banned drugs, exempted drugs, and absence of Codex standards, providing a more complete assessment of the regulatory environment in the livestock, aquaculture, and dairy markets.
Data
The analysis draws on FoodChain ID Inc.’s 2020 global MRL dataset, which is then cleaned, formatted, and matched with 2020 U.S. export data ($USm) from the USDA Foreign Agricultural Service (FAS). In 2020, the top 10 U.S. destinations for beef, poultry, swine, and dairy (milk) products accounted for 92%, 70%, 93% and 71% of respective exports – by value.
Regulatory Stringency for Top Export Destinations for U.S. Beef, Pork, and Poultry
We assess the regulatory stringency between the United States and its top 10 export destinations for beef, poultry, swine, and dairy (milk) products, focusing on potential protectionism in these key markets. Using the MRL-factor-based index developed by Okunola et al. (2025), we construct both Codex-based and bilateral stringency differences for the U.S. and these major export markets. This stringency index ranges from negative infinity to unity. A negative index indicates regulatory laxity. A positive index signifies higher stringency, and a zero index reflects harmonized MRLs between national and Codex MRLs. The bilateral stringency divergence is estimated as the difference between Codex-based aggregate indices of the U.S. and its export destinations.
Relative to CODEX
The regulatory comparison of U.S. MRLs to those of its major trading partners with Codex standards is presented in Table 1.
- Beef: The U.S. score is −3.00, meaning that on average, U.S. veterinary drug MRLs in beef are laxer than Codex standards. Major markets like the European Union (EU) (0.14), Hong Kong (0.04), and Mexico (0.03) adopt standards stricter than Codex.
- Poultry: The U.S. score is –9.07, meaning that on average, U.S. veterinary drug MRLs in poultry are laxer than Codex standards. All major partners (Mexico, China, Guatemala, and others) maintain stricter or Codex-compliant policies.
- Swine: The U.S. deviation is −3.05, indicating that its pork MRLs are, on average, less stringent than Codex standards. Several key trading partners enforce tighter limits: China (−0.47), Japan (−1.64), and Canada (−1.00). Latin American partners like Chile and Colombia generally align with Codex. Australia's laxity (−20.62) is a notable high.
- Dairy (Milk): The U.S. score is –3.87, meaning that on average, U.S. veterinary drug MRLs in dairy milk are laxer than Codex standards. Export markets such as Mexico, China, and Vietnam set tighter or Codex-aligned standards. Although some (e.g., Indonesia, Taiwan) fall short of Codex, they are closer than the U.S.
Regulatory Stringency for Top Export Destinations for U.S. Beef, Pork, and Poultry
The bilateral regulatory comparison of U.S. MRLs with those of its major trading partners is presented in Table 1.
Relative to the United States
- Beef: U.S. MRLs for veterinary drugs are generally more lenient than those of its top beef export destinations. Among the ten largest markets, U.S. standards are less stringent in nine, with the exception of Indonesia (13.12).
- Poultry: U.S. MRLs are consistently more lenient than those of key poultry export destinations. The gap is especially wide in major markets such as China (–8.97), Mexico (–9.06), and Taiwan (–7.81).
- Swine: In the swine sector, U.S. MRLs are also generally less stringent. Of the top ten export destinations, only Australia (17.57) maintains laxer standards.
- Dairy (Milk): U.S. MRLs for dairy products are more lenient across all major export markets. Each of the top ten destinations shows a negative bilateral stringency score, with the smallest gap observed in Indonesia (–1.24).
Conclusion
The findings highlight significant regulatory divergence in the veterinary drug MRLs between the U.S. and its major trading partners in major international meat and dairy markets, especially in the poultry sector. This suggests a difference in U.S. domestic regulatory standards, Codex standards, and foreign market expectations. While these gaps do not necessarily indicate protectionist intent, they increase the risk of MRL-related trade frictions, especially if trade tensions escalate or importing countries tighten enforcement. U.S. exporters, therefore, face an elevated risk of encountering non-tariff regulatory challenges that could disrupt market access.
To mitigate these risks, it is essential for U.S. meat and dairy producers to understand the regulatory environments in major export destinations. A nationwide outreach program could provide technical support to help U.S. producers navigate diverse MRL standards. Additionally, relevant trade agencies could engage in bilateral and multilateral dialogues to reduce potential MRL-related trade barriers. Establishing a system to monitor MRL discrepancies across key markets would allow for proactive management of risks, guiding decisions on standard-setting and improving the overall resilience of U.S. agricultural exports.
References
FAO and WHO. Food and Agriculture Organization and World Health Organization. Principles and methods for the risk assessment of chemicals in food. World Health Organization, 2009. iris.who.int/bitstream/handle/10665/44065/?sequence=9
Disdier, Anne-Célia, and Stéphan Marette. 2010. “The Combination of Gravity and Welfare Approaches for Evaluating Nontariff Measures.” American Journal of Agricultural Economics 92 (3): 713–26. https://doi.org/10.1093/ajae/aaq026.
Disdier, Anne‐Célia, and Frank Van Tongeren. 2010. “Non‐Tariff Measures in Agri‐Food Trade: What Do the Data Tell Us? Evidence From a Cluster Analysis on OECD Imports.” Applied Economic Perspectives and Policy 32 (3): 436–55. https://doi.org/10.1093/aepp/ppq008.
Gourdon, Julien, Susan Stone, and Frank Van Tongeren. 2020. “Non-tariff Measures in Agriculture.” OECD Food, Agriculture and Fisheries Working Papers, November. https://doi.org/10.1787/81933f03-en.
Grant, Jason, Everett Peterson, and Radu Ramniceanu. 2015. “Assessing the Impact of SPS Regulations on U.S. Fresh Fruit and Vegetable Exports.” DOAJ (DOAJ: Directory of Open Access Journals), January. https://doi.org/10.22004/ag.econ.197381.
Hejazi, Mina, Jason H. Grant, and Everett Peterson. 2021. “Trade Impact of Maximum Residue Limits in Fresh Fruits and Vegetables.” Food Policy 106 (December): 102203. https://doi.org/10.1016/j.foodpol.2021.102203.
Karemera, David, Bo Xiong, Gerald Smalls, and Louis Whitesides. 2021. “The Political Economy of Maximum Residue Limits: A Long‐term Health Perspective.” Journal of Agricultural Economics 73 (3): 709–19. https://doi.org/10.1111/1477-9552.12476.
Li, Yuan, and John C. Beghin. 2011. “A Meta-analysis of Estimates of the Impact of Technical Barriers to Trade.” Journal of Policy Modeling 34 (3): 497–511. https://doi.org/10.1016/j.jpolmod.2011.11.001.
Okunola, Akinbode, Elliott Dennis, and John Beghin. 2025. “Are Veterinary Drug Maximum Residue Limits Protectionist? International Evidence.” Applied Economic Perspectives and Policy, March. https://doi.org/10.1002/aepp.13516.
Santeramo, Fabio Gaetano, and Emilia Lamonaca. 2019. “The Effects of Non‐tariff Measures on Agri‐food Trade: A Review and Meta‐analysis of Empirical Evidence.” Journal of Agricultural Economics 70 (3): 595–617. https://doi.org/10.1111/1477-9552.12316.
Yeung, May T., William A. Kerr, Blair Coomber, Matthew Lantz, and Alyse McConnell. 2017. Declining International Cooperation on Pesticide Regulation. Springer eBooks. https://doi.org/10.1007/978-3-319-60552-4.
Table 1. Aggregate regulatory stringency scores.
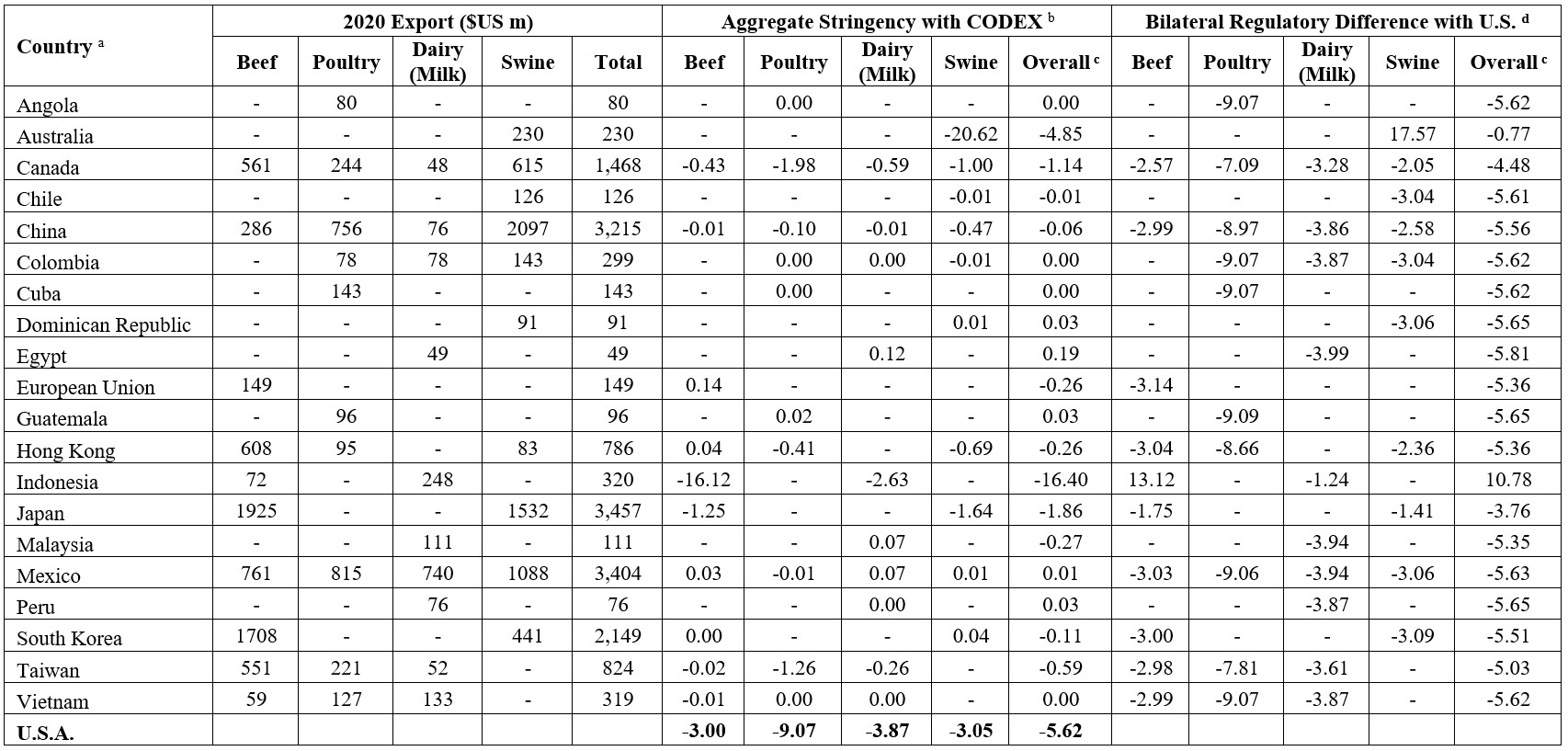
Source: Authors’ computation. a The analysis includes only the top 10 U.S. export destinations for each product market (beef, poultry, dairy, and swine). Blank cells indicate that the country is not a top 10 destination for the specified market, and thus, no regulatory stringency score is reported. b Aggregate stringency index values interpret as follows: zero indicates overall compliance with Codex MRLs; positive values reflect stricter national standards; and negative values reflect overall regulatory laxity relative to Codex. c Overall stringency reflects each country’s aggregate regulatory stance on veterinary drug MRLs across all three markets. d Bilateral regulatory stringency is the difference in the Codex-based regulatory indices relative to the U.S. index for each export destination. Zero bilateral stringency index indicates that both U.S. and its export destination have MRLs that are aligned with Codex MRLs. A positive bilateral regulatory stringency indicates that relative to Codex, U.S. MRLs are on aggregate more stringent than its export partner, while a negative difference indicates U.S. MRLs are laxer than those of the export destination.
Elliott Dennis
Associate Professor
Department of Agricultural Economics
University of Nebraska – Lincoln
elliott.dennis@unl.edu
Akinbode Okunola
Graduate Research Assistant
Department of Agricultural Economics
University of Nebraska – Lincoln
aokunola2@huskers.unl.edu
